The world’s first cloned pig operated by a robot was born in Tianjin
The graph is based on the minimum forcerobotSomatic cell nuclear transfer and cloned piglets. (Photo courtesy of Nankai University) Photo by Zhong Xin
OFweek Robot Net News According to a report from Chinanews.com Tianjin on July 3, recently, after a long wait of more than two months, a special “paternity test” report was released, and 13 cloned piglets were not related to the “surrogate” mother. , and only has a “parent-child relationship” with the donor cell. This is medically proved that the world’s first robotically operated somatic cell cloned pig was born in Tianjin, China.
After 110 days of gestation, on April 26 and 29, two ordinary “surrogate” sows gave birth to 13 healthy purebred landrace pigs.Compared with the previous “manual operation” cloning technology, the robot’sautomation“Operation”, with less force, less damage to cells, and higher precision operation increased the “blastocyst rate”, a key indicator of the success of somatic cell cloning technology, from 10% to 20%. This data surprised global peers.
The research on “Robot Manipulation of Somatic Cell Cloning Pigs” comes from an interdisciplinary research team led by Professor Zhao Xin from the Institute of Robotics of Nankai University. Tianjin Institute of Animal Husbandry and Veterinary Medicine is the main cooperative unit. The “Micro-manipulation Robot System for Biomedical Engineering” developed by the Robotics Institute of Nankai University for many years won the second prize of the 2002 National Technological Invention Award. The birth of the cloned pig is also another major breakthrough in the technology after 15 years.
Somatic cell cloning is one of the classic methods for improving biological varieties. It removes the nuclei of common oocytes and injects them into somatic cells of superior varieties. The advantage of this method is that the progeny obtained must be of an elite variety. However, the success rate is extremely low, which has become a bottleneck in the development of somatic cell cloning technology.
“As one of the most complex micro-operations, somatic cell cloning technology urgently needs to be roboticized. Through Robotic somatic cell nuclear transfer operations, the success rate of subsequent cell culture and thus the success rate of the entire somatic cell cloning technology is improved, which is the real challenge we face. Challenge.” Zhao Xin said.
In response to the above problems, the research team of Nankai University has carried out scientific research to meet the needs of life science development, and developed an in-situ microscopic analysis and operation instrument with visualization, minimally invasive, fixed-point and quantitative functions, integrating detection, analysis and operation. Using this instrument, a robotic cell nuclear transfer procedure was realized.
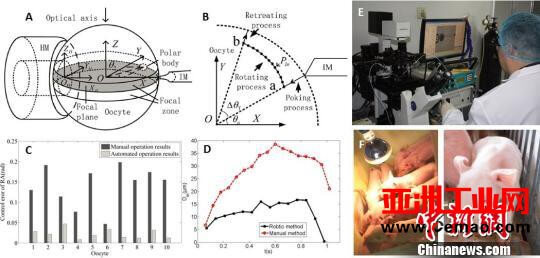
According to reports, the key difficulty of somatic cell cloning technology based on robotic micromanipulation is how to minimize the damage to cells. The Nankai University team designed and implemented a robotic cell nuclear transfer process, including three parts: oocyte pulling to find polar bodies, nuclear extraction and somatic cell injection.
By analyzing the cell force during the contact between the micro-manipulation tool and the cell, the researchers realized the minimum force-based cell toggle and nucleation respectively, which ensured the minimum force on the cell during the nuclear transfer operation. “By manually operating the cells, the maximum deformation of the cells is 30 to 40 microns. After calculation, the maximum deformation of the cells operated by the robot is reduced to 10 to 15 microns. If the previous force was a ‘single punch’, now it is a ‘soft punch’. Push’.” Zhao Xin introduced that the micro-operating system adopts the operation based on the equilibrium pressure model, which also makes the process of extracting the nucleus more “controllable” and “gentle”.
The experimental results showed that the minimal force-based cell plucking method significantly reduced the damage to the cells; the subsequent cell culture showed that compared with the manual operation, the minimal force-based cell nucleation operation resulted in a significantly higher subsequent cell development rate.
“The most exciting thing is that we used this micro-manipulation platform to perform nuclear transfer of 53 oocytes in in vitro experiments, of which 11 reconstructed embryos developed into blastocysts that marked the success of cloning, with a development rate of 21%. However, the blastocyst rate of the previous somatic cell clone has always been maintained at about 10%.” Zhao Xin said.
In order to make this technology “bloom and bear fruit”, the scientific research team of Nankai University applied the nuclear transfer method to the whole process of cloning pigs, and completed thousands of nuclear transfer operations successively to verify the effectiveness of the method. In early January 2017, 510 nuclear transfer operations were completed in four batches, and the 510 cloned embryos were transferred into 6 sows. In the end, the two sows were successfully conceived and gave birth to 7 and 6 pigs respectively on April 26 and 29, totaling 13 healthy cloned pigs.
“Our study is the first to guide micromanipulation from the developmental perspective of cells, linking the micromanipulation process with cell developmental outcomes through cell forces. The generalization of this method can further improve the contribution of micromanipulation technology to the overall biological process. It is foreseen that it has good application prospects in the fields of assisted reproduction, animal and plant variety improvement, public medical care, and livestock production.” Zhao Xin said.
It is understood that the project has been supported by the national major scientific research instrument development project, the “863” plan advanced manufacturing field, and the Tianjin intelligent robot major science and technology project.
The Links: IGBT ELECTRONIC
Pre: Teledyne Introduces Contact Image Sen... Next: YOFC’s 2016 smart manufacturing...




